INTRODUCTION
The laboratory studies circuits, synapses and information processing in cerebral cortex. We focus on the rodent’s primary somatosensory (S1) cortex, which is a major model system for studying sensory information processing, learning, and plasticity in cerebral cortex. S1 processes tactile (touch) information from the rodent’s facial whiskers, which are active tactile detectors analogous to human fingertips. Cell types and circuits in rodent S1 are similar to primates. Thus, studying rodent S1 can reveal the underlying principles of cortical function across mammals, including humans.
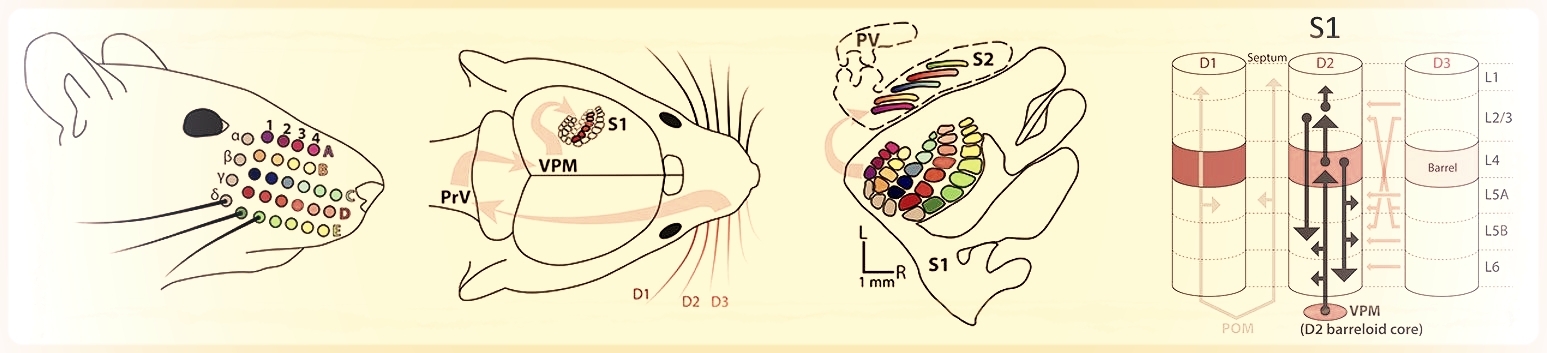
Sensory information from the whiskers is relayed to S1 cortex, which contains a macroscopic whisker map. In S1, specific neural circuits couple neurons within and across cortical columns.
Closeup view of a mouse whisking on a raised grating with C1 and C2 whiskers. Purple line shows imaging plane for high-speed whisker tracking. (Brian Isett)
Neuronal responses to deflections of single whiskers (indicated by flashing square) visualized using 2-photon calcium imaging. (Amy LeMessurier)
Our goal is to identify major principles of cortical information processing, information storage and learning, from synapse to circuit to neural systems levels. We study how synapse function impacts circuit-level processing; how recent sensory experience alters S1 neurons and circuits to store sensory information and optimize cortical processing; and how homeostatic plasticity acts to maintain normal circuit function despite changing sensory input. We study how sensory information is organized within precise micro-scale maps, and how whisker information is transformed across cortical layers. We study how real-world tactile information is encoded by population activity in S1, to guide perception and behavior.
Our research will provide much-needed basic knowledge about brain function, and will enable better understanding of common disorders of cortical function and plasticity, including autism and epilepsy. We are actively investigating the neural circuit basis for autism, using transgenic mouse models of this disorder.
AREAS OF CURRENT RESEARCH
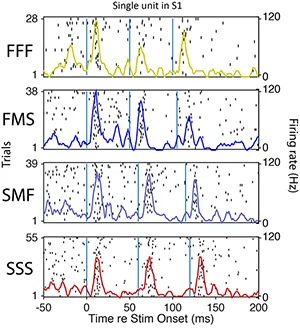
Spiking of a S1 single unit to temporal patterns of whisker deflection (Leah McGuire).
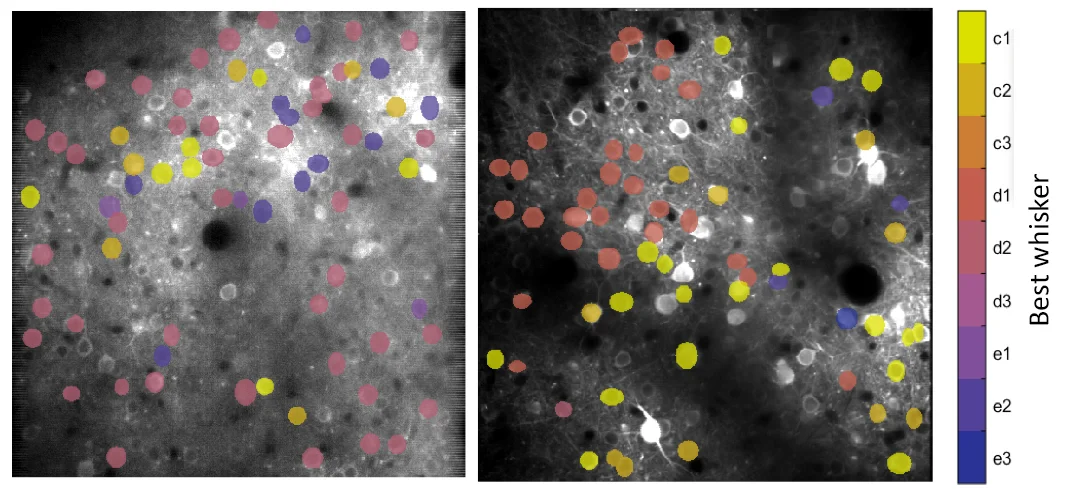
Neural coding and information processing in whisker somatosensory cortex. How do cortical circuits extract and represent sensory information? What is the role of the different cortical layers in this process? What computations are implemented by sensory cortex? To address these questions, we study how tactile information is sensed by moving whiskers and processed and encoded in S1 cortex. Methods include multi-site neural recordings during quantitative sensory behavior, 2-photon calcium imaging of neural activity in S1, optogenetics, and computational analysis of population coding. We also study the precise structure of sensory maps, how they vary across layers, and how learning is implemented by changes in maps.
Micro-scale organization of the whisker map, in which nearby cells in L2/3 of S1 cortex are tuned for different whiskers in a salt-and-pepper intermixing. Derived from 2-photon Ca imaging data.
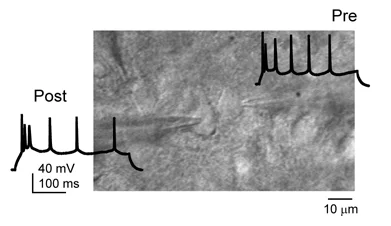
Paired whole-cell recording from synaptically connected pyramidal neurons in L2/3 of S1.
Synaptic mechanisms for cortical map plasticity and stability. Information is stored in neural networks by Hebbian synaptic plasticity, as well as by anatomical rewiring of cortical microcircuits and other mechanisms. We work to identify specific sites and mechanisms of plasticity, and to understand how multiple plasticity mechanisms interact to drive overall changes in receptive fields and maps. Methods include slice physiology, whole-cell patch clamp recording, optogenetics, and calcium imaging. Many of our current studies focus on plasticity in inhibitory microcircuits, and how it contributes to plasticity and stability in cortical maps.
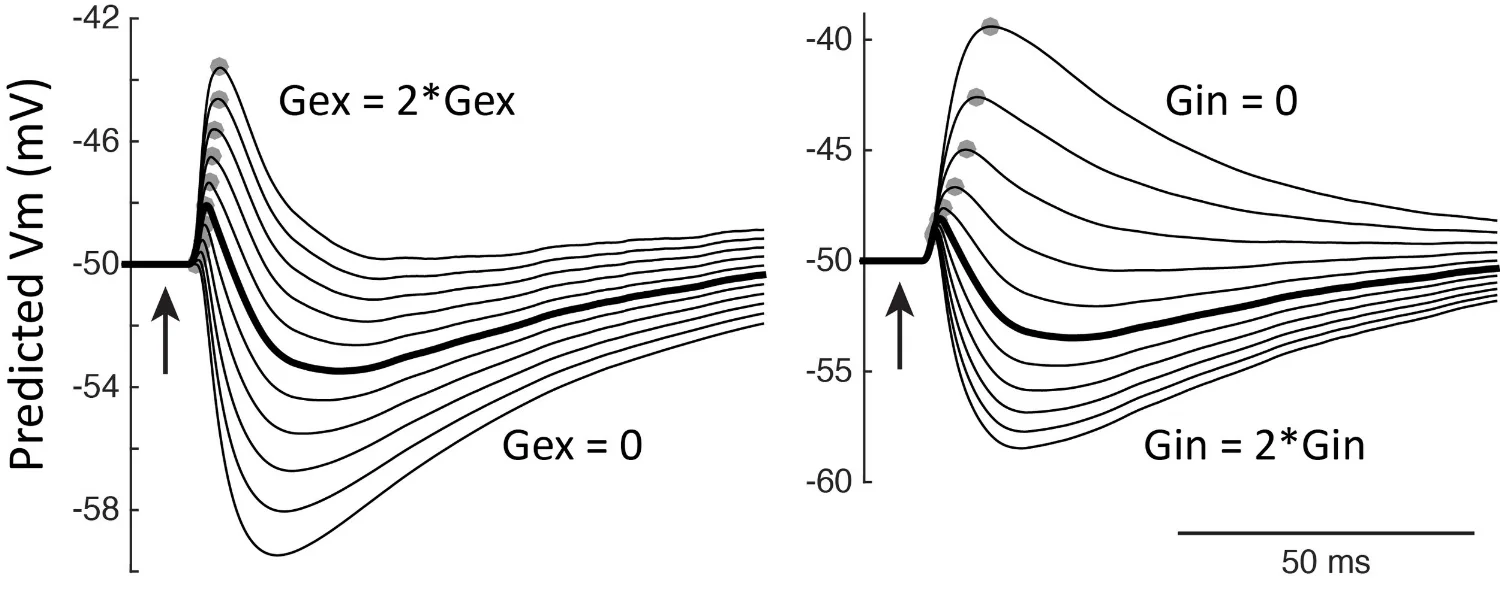
Neural circuit basis for autism spectrum disorders. Autism is a family of neurodevelopmental disorders with a complex genetic basis, and is often associated with tactile sensory phenotypes. Using transgenic mouse models of monogenic forms of autism, we study how synapse- and circuit-level function is disrupted in cerebral cortex in autism. We study how this affects neural coding and information processing, and test whether different genetic forms of autism share common physiological phenotypes in cortex. If so, it may be possible to develop therapeutic interventions that are broadly effective across multiple genetic types of autism.
Modeling EPSPs and IPSPs to understand how excitation-inhibition ratio affects neural excitability in autism. (Antoine, Langberg, Schnepel et al., Neuron 2019).